Which Of The Following Is Not A Chemical Change?
7.two: Evidence of a Chemic Reaction
- Page ID
- 47498
Learning Objectives
- Identify the evidence for chemic reactions.
In a chemical change, new substances are formed. In social club for this to occur, the chemical bonds of the substances suspension, and the atoms that compose them separate and rearrange themselves into new substances with new chemical bonds. When this process occurs, we phone call it a chemical reaction. A chemical reaction is the process in which one or more substances are inverse into i or more new substances.
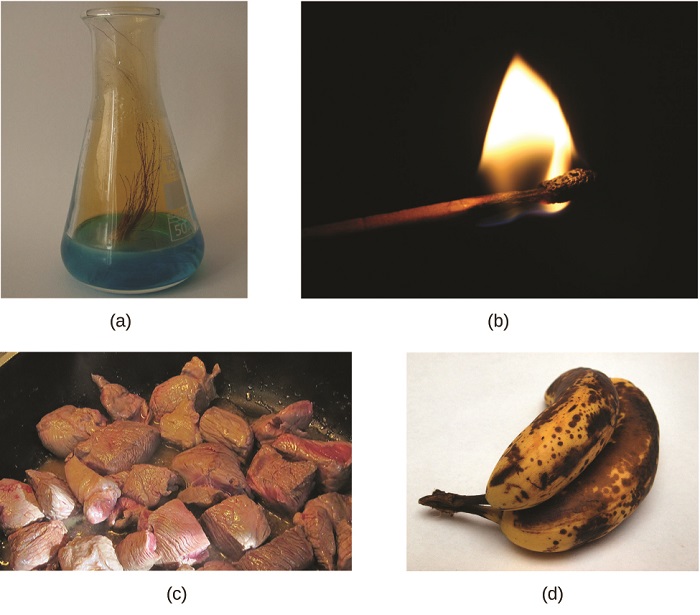
To place a chemic reaction, we look for a chemical change. A chemical change always produces one or more types of affair that differ from the matter nowadays before the modify. The formation of rust is a chemical change because rust is a dissimilar kind of thing than the iron, oxygen, and water present before the rust formed. The explosion of nitroglycerin is a chemic alter considering the gases produced are very unlike kinds of matter from the original substance. Other examples of chemical changes include: reactions that are performed in a lab (such as copper reacting with nitric acrid), all forms of combustion (called-for), and nutrient being cooked, digested, or rotting (Figure \(\PageIndex{1}\)).
Video\(\PageIndex{1}\): Bear witness of a Chemic Reaction
Instance \(\PageIndex{1}\): Evidence of a Chemical Reaction
Which of the following is a chemical reaction?
- Freezing liquid mercury.
- Calculation xanthous to blue to brand green.
- Cutting a piece of paper into two pieces.
- Dropping a sliced orange into a vat of sodium dydroxide.
- Filling a balloon with natural air.
Solution
A, B, C, & E involve only physical changes. A sliced orange has acid (citric acid) that tin react with sodium hydroxide, and then the answer is D.
Exercise \(\PageIndex{1}\)
Which of the following is a chemical reaction?
- Painting a wall bluish.
- A bicycle rusting.
- Ice cream melting.
- Scratching a key across a desk-bound.
- Making a sand castle.
- Answer
-
B
Example \(\PageIndex{two}\): Evidence of a Chemical Reaction
Which of the post-obit is not a chemical reaction?
- Shattering drinking glass with a baseball.
- Corroding metallic.
- Fireworks exploding.
- Lighting a match.
- Baking a cake.
Solution
Shattering glass with a baseball results in drinking glass cleaved into many pieces but no chemical change happens, so the answer is A.
Exercise \(\PageIndex{2}\)
Which of the post-obit is Not a chemical reaction?
- Frying an egg.
- Slicing carrots.
- A Macbook falling out of a window.
- Creating ATP in the homo body.
- Dropping a fizzy tablet into a drinking glass of water.
- Respond
-
B and C
Summary
Chemical reactions tin exist identified via a broad range of different appreciable factors including change in color, energy change (temperature change or light produced), gas production, formation of precipitate and change in properties.
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map:_Introductory_Chemistry_%28Tro%29/07:_Chemical_Reactions/7.02:_Evidence_of_a_Chemical_Reaction
Posted by: phillipsnobjess76.blogspot.com


0 Response to "Which Of The Following Is Not A Chemical Change?"
Post a Comment