What type of properties can be observed and measured without changing the substance?
Affiliate 1. Essential Ideas
1.3 Physical and Chemical Properties
Learning Objectives
By the end of this section, you will exist able to:
- Identify properties of and changes in thing every bit physical or chemic
- Identify properties of matter every bit extensive or intensive
The characteristics that enable us to distinguish one substance from another are called properties. A physical property is a characteristic of matter that is not associated with a change in its chemical composition. Familiar examples of physical properties include density, color, hardness, melting and boiling points, and electrical conductivity. Nosotros tin can notice some physical properties, such equally density and color, without irresolute the concrete land of the matter observed. Other physical properties, such as the melting temperature of iron or the freezing temperature of water, tin can only be observed as affair undergoes a physical change. A physical alter is a change in the state or backdrop of matter without any accompanying change in its chemical limerick (the identities of the substances contained in the matter). We find a physical modify when wax melts, when sugar dissolves in coffee, and when steam condenses into liquid water (Figure 1). Other examples of physical changes include magnetizing and demagnetizing metals (as is done with common antitheft security tags) and grinding solids into powders (which can sometimes yield noticeable changes in color). In each of these examples, there is a modify in the concrete state, form, or properties of the substance, only no modify in its chemical composition.

The change of one type of matter into another type (or the inability to change) is a chemical holding. Examples of chemical backdrop include flammability, toxicity, acidity, reactivity (many types), and oestrus of combustion. Iron, for case, combines with oxygen in the presence of water to form rust; chromium does not oxidize (Figure 2). Nitroglycerin is very dangerous considering it explodes easily; neon poses virtually no hazard because information technology is very unreactive.

To identify a chemical property, we wait for a chemical alter. A chemical change always produces one or more than types of matter that differ from the affair sspresent before the alter. The germination of rust is a chemic change because rust is a different kind of affair than the iron, oxygen, and water present before the rust formed. The explosion of nitroglycerin is a chemical change because the gases produced are very different kinds of thing from the original substance. Other examples of chemical changes include reactions that are performed in a lab (such as copper reacting with nitric acid), all forms of combustion (called-for), and food being cooked, digested, or rotting (Figure 3).
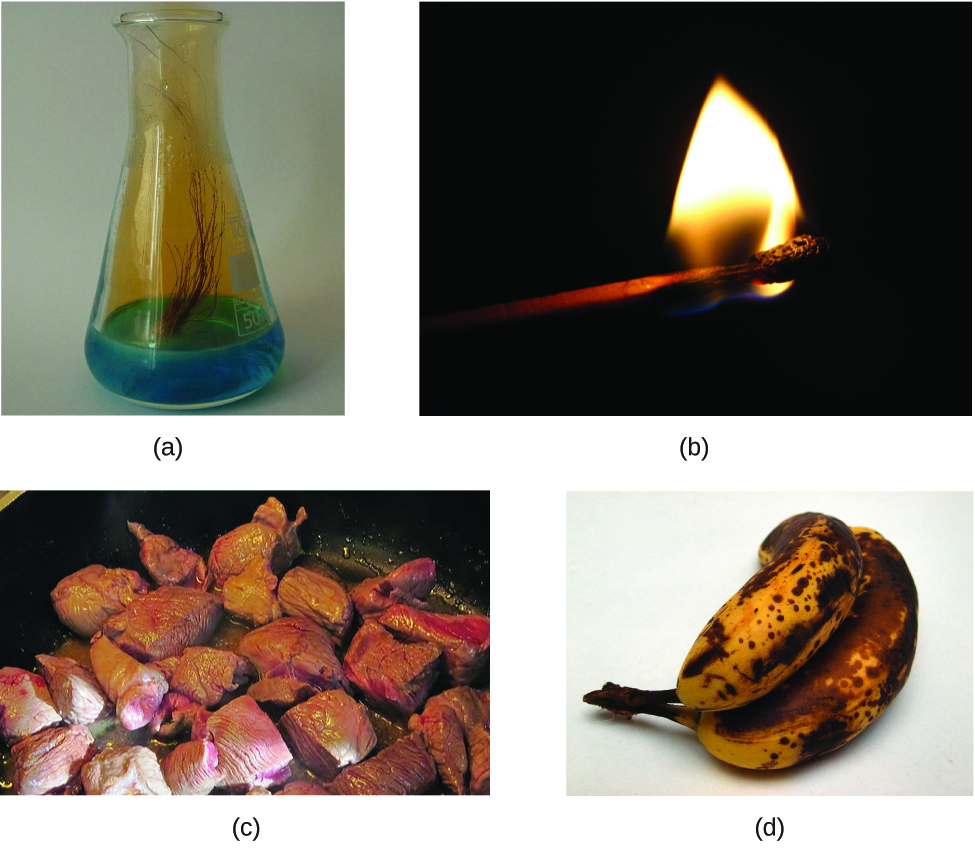
Properties of thing autumn into one of 2 categories. If the belongings depends on the amount of thing present, information technology is an extensive property. The mass and book of a substance are examples of extensive properties; for instance, a gallon of milk has a larger mass and book than a cup of milk. The value of an all-encompassing property is directly proportional to the amount of matter in question. If the holding of a sample of affair does not depend on the amount of thing present, it is an intensive belongings. Temperature is an case of an intensive property. If the gallon and cup of milk are each at 20 °C (room temperature), when they are combined, the temperature remains at 20 °C. As another example, consider the distinct but related properties of heat and temperature. A driblet of hot cooking oil spattered on your arm causes brief, pocket-size discomfort, whereas a pot of hot oil yields astringent burns. Both the drop and the pot of oil are at the same temperature (an intensive belongings), but the pot clearly contains much more heat (extensive property).
Take chances Diamond
You may have seen the symbol shown in Effigy 4 on containers of chemicals in a laboratory or workplace. Sometimes chosen a "burn down diamond" or "hazard diamond," this chemic hazard diamond provides valuable information that briefly summarizes the various dangers of which to exist aware when working with a particular substance.
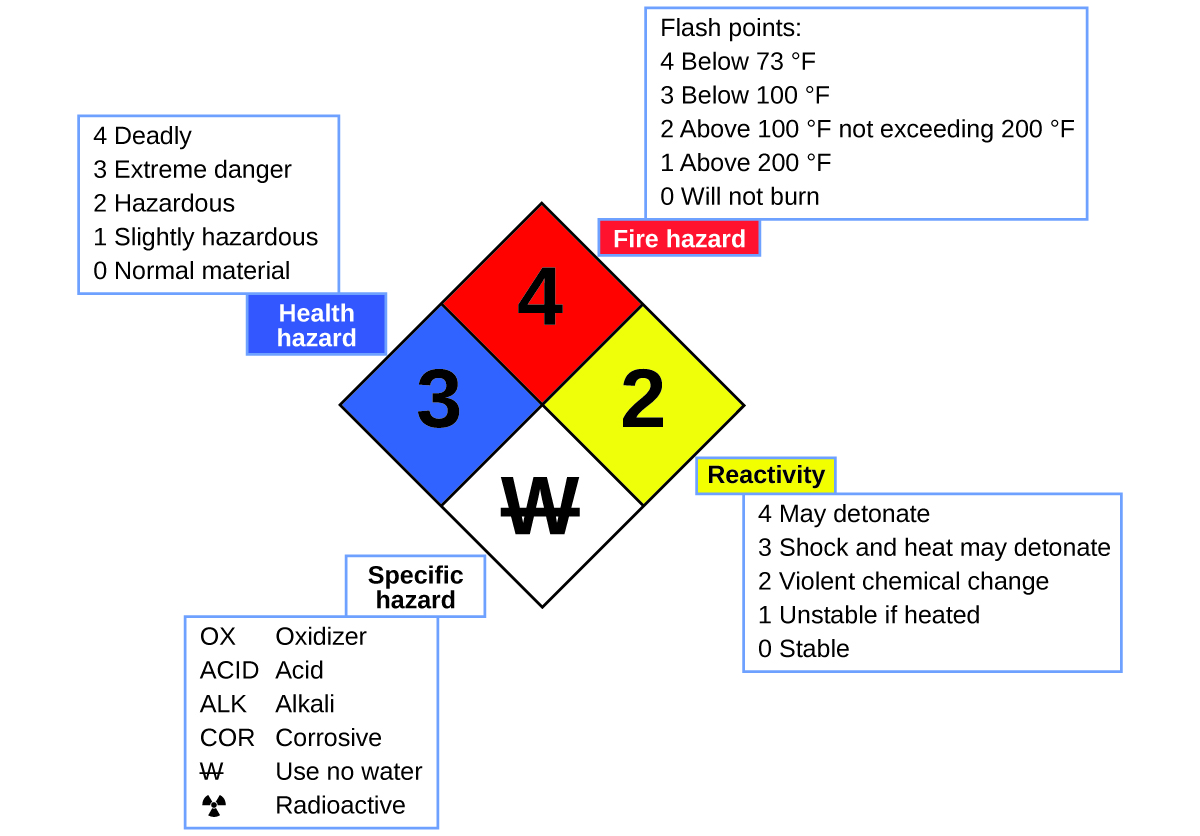
The National Burn Protection Bureau (NFPA) 704 Adventure Identification Organisation was developed by NFPA to provide safety information nearly certain substances. The system details flammability, reactivity, health, and other hazards. Within the overall diamond symbol, the top (ruby-red) diamond specifies the level of fire hazard (temperature range for wink point). The bluish (left) diamond indicates the level of wellness gamble. The yellowish (right) diamond describes reactivity hazards, such as how readily the substance volition undergo detonation or a violent chemical modify. The white (lesser) diamond points out special hazards, such as if it is an oxidizer (which allows the substance to burn in the absenteeism of air/oxygen), undergoes an unusual or dangerous reaction with water, is corrosive, acidic, alkaline, a biological hazard, radioactive, and so on. Each hazard is rated on a scale from 0 to 4, with 0 beingness no hazard and 4 being extremely hazardous.
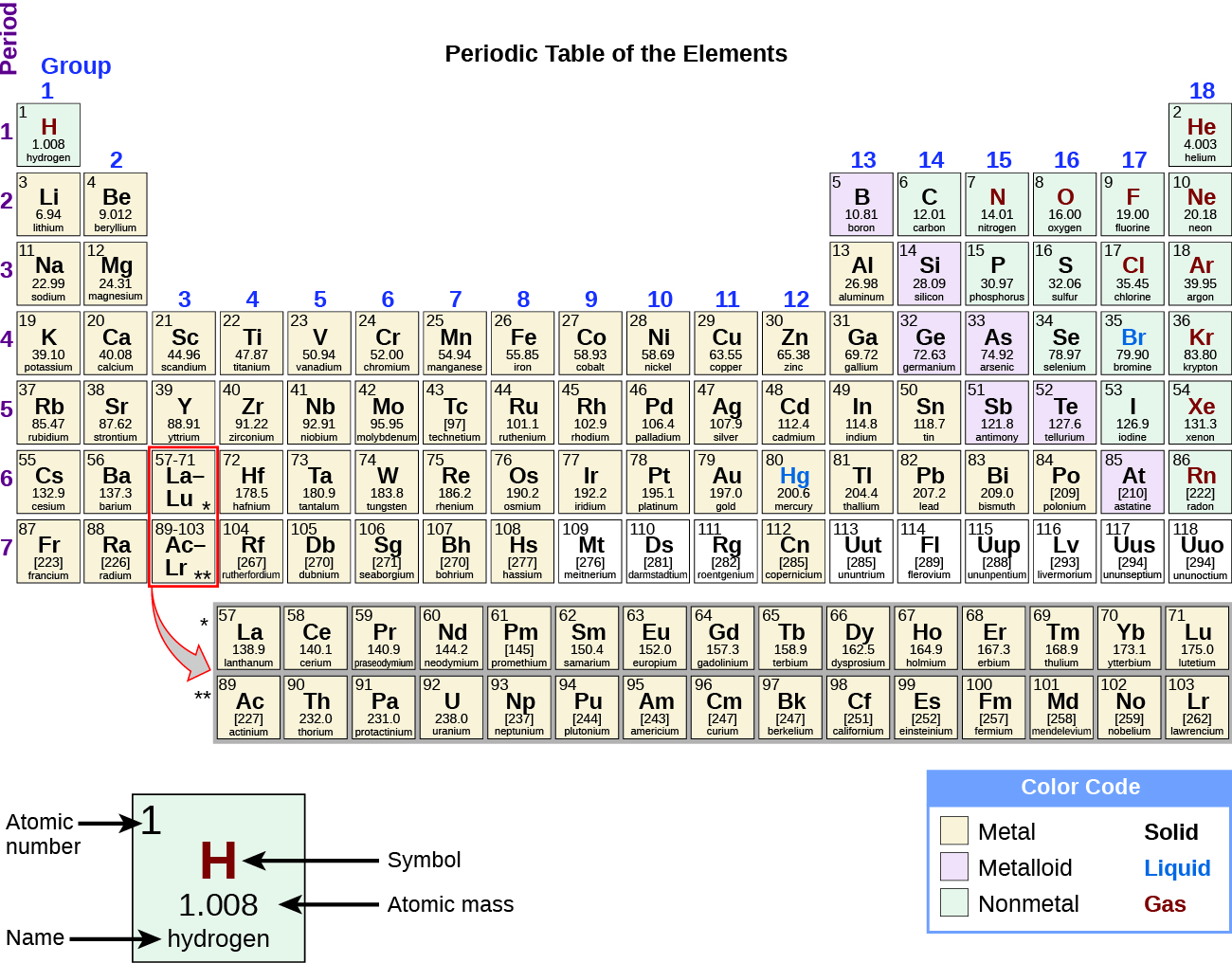
While many elements differ dramatically in their chemical and concrete backdrop, some elements have similar properties. We can identify sets of elements that showroom mutual behaviors. For example, many elements carry estrus and electricity well, whereas others are poor conductors. These properties can exist used to sort the elements into three classes: metals (elements that deport well), nonmetals (elements that bear poorly), and metalloids (elements that have properties of both metals and nonmetals).
The periodic table is a table of elements that places elements with similar properties close together (Figure 4). Yous will learn more about the periodic table as you lot continue your written report of chemical science.
Primal Concepts and Summary
All substances have distinct physical and chemical properties, and may undergo physical or chemical changes. Physical backdrop, such as hardness and boiling point, and physical changes, such as melting or freezing, practice not involve a alter in the composition of matter. Chemical properties, such flammability and acidity, and chemical changes, such as rusting, involve production of affair that differs from that present beforehand.
Measurable properties fall into one of ii categories. All-encompassing properties depend on the corporeality of affair present, for example, the mass of gold. Intensive properties do non depend on the amount of thing nowadays, for example, the density of aureate. Oestrus is an example of an extensive belongings, and temperature is an instance of an intensive property.
Chemistry Cease of Chapter Exercises
- Allocate the six underlined properties in the following paragraph as chemic or concrete:
Fluorine is a stake xanthous gas that reacts with near substances. The gratis chemical element melts at −220 °C and boils at −188 °C. Finely divided metals burn down in fluorine with a bright flame. Xix grams of fluorine will react with i.0 gram of hydrogen.
- Classify each of the following changes as physical or chemical:
(a) condensation of steam
(b) called-for of gasoline
(c) souring of milk
(d) dissolving of saccharide in water
(due east) melting of aureate
- Classify each of the post-obit changes every bit physical or chemic:
(a) coal called-for
(b) ice melting
(c) mixing chocolate syrup with milk
(d) explosion of a firecracker
(e) magnetizing of a screwdriver
- The volume of a sample of oxygen gas changed from ten mL to 11 mL as the temperature changed. Is this a chemical or concrete change?
- A ii.0-liter volume of hydrogen gas combined with 1.0 liter of oxygen gas to produce two.0 liters of water vapor. Does oxygen undergo a chemic or concrete change?
- Explain the difference between extensive backdrop and intensive properties.
- Identify the following properties every bit either extensive or intensive.
(a) book
(b) temperature
(c) humidity
(d) heat
(east) boiling point
- The density (d) of a substance is an intensive property that is defined every bit the ratio of its mass (m) to its book (5).
[latex]\text{density}= \frac{\text{mass}}{\text{volume}}[/latex] [latex]\text{d} = \frac{\text{1000}}{\text{V}}[/latex]
Considering that mass and book are both extensive properties, explain why their ratio, density, is intensive.
Glossary
- chemical change
- change producing a different kind of matter from the original kind of matter
- chemical property
- beliefs that is related to the change of one kind of matter into another kind of matter
- extensive property
- property of a substance that depends on the amount of the substance
- intensive property
- property of a substance that is contained of the amount of the substance
- physical alter
- alter in the state or properties of matter that does non involve a modify in its chemical composition
- physical property
- characteristic of matter that is not associated with whatsoever change in its chemical composition
Solutions
Answers for Chemistry End of Chapter Exercises
two. (a) physical; (b) chemic; (c) chemical; (d) concrete; (e) physical
4. concrete
6. The value of an extensive holding depends upon the amount of matter being considered, whereas the value of an intensive property is the same regardless of the amount of matter being considered.
8. Beingness extensive properties, both mass and book are direct proportional to the amount of substance nether study. Dividing one extensive holding by another will in effect "abolish" this dependence on corporeality, yielding a ratio that is independent of amount (an intensive property).
Source: https://opentextbc.ca/chemistry/chapter/physical-and-chemical-properties/
Posted by: phillipsnobjess76.blogspot.com


0 Response to "What type of properties can be observed and measured without changing the substance?"
Post a Comment